A
Abnormal Toxicity Test, 521
ABO Group of Donors, Determination of, 692
Acetate Buffer pH 3.0, 330
Acetate Buffer pH 3.5, 330
Acetate Buffer pH 4.4, 330
Acetate Buffer pH 4.6, 330
Acetate Buffer pH 6.0, 330
Acetic Acid, 294
Acetic Acid, Dilute, 294
Acetic Acid, Glacial, 294
Acetic Acid, Glacial, Anhydrous, 294
Acetic-Ammonia Buffer pH 3.7, Ethanolic, 330
Acetic Anhydride, 294
Acetic Anhydride-Pyridine TS, 331
Acetic-Bromine TS, 331
Acetylacetone, 294
N-Acetylneuraminic Acid, 294
Acid Black 1, 310
Acid Blue 74, 327
Acid Blue 83, 294
Acid Blue 147, 322
Acid-insoluble Ash, 518
Acid-neutralizing Capacity, 483
Acid Orange 6, 330
Acid Red 87, 303
Acid Value, 480
Acknowledgement, xxii
Acrylamide, 294
Acyclovir, 21
Acyclovir Tablets, 22
Added Substance, General Notices, 6
Addition Information, General Notices, 9
Adsorbed Diphtheria Vaccine, Biological Assay of, 708
Adsorbed Pertussis Vaccine, Biological Assay of, 709
Adsorbed Tetanus Vaccine, Biological Assay of, 710
Agar, 294
Agarose for Chromatography, Cross-linked, 294
Agarose for Chromatography 1, Cross-linked, 294
Albumin, Bovine Serum, 295
Albumin, Human, 295
Albumin Solution, 194
Aldehyde-free Alcohol, 304
Aldehydes, Determination of, 519
Alizarin Complexone Dihydrate, 295
Alizarin Complexone Dihydrate TS, 331
Alizarin Fluorine Blue, 295
Alizarin Red S, 326
Alizarin Red TS, 326
Alizarin S, 326
Alizarin Yellow GG, 326
Alternative Fluid Thioglycolate Medium, 615
Alum, 295
Aluminium, Limit Tests for, 474
Aluminium Nitrate, 295
Aluminium Oxide, Activated Acid, 295
Aluminium Potassium Sulfate, 295
Aluminium Standard Solution (2 ppm Al), 325
Aluminium Standard Solution (10 ppm Al), 325
Amido Black 10B, 310
4-Aminoantipyrine, 295
2-Amino-2-methylpropane, 302
4-Aminophenazone, 295
Aminophenazone TS, 331
Aminopyrazolone, 295
Aminopyrazolone TS, 331
Ammonia, 295
Ammonia Buffer pH 10.0, 330
Ammonia Solution, Dilute, 295
Ammonia Solution, Strong, 295
Ammonia TS, 331
Ammonium, Limit Tests for, 475
Ammonium Acetate, 295
Ammonium Chloride, 295
Ammonium Chloride TS, 331 Ammonium Dihydrogenphosphate, 295
Ammonium Iron(III) Sulfate, 295 Ammonium Iron(III) Sulfate TS, 331
Ammonium Mercurithiocyanate TS, 331
Ammonium Metavanadate, 295
Ammonium Molybdate, 295
Ammonium Molybdate TS, 331
Ammonium Molybdate with Ascorbic Acid TS, 331
Ammonium Nitrate, 295
Ammonium Oxalate, 295
Ammonium Peroxydisulfate, 296
Ammonium Persulfate, 296
Ammonium Phosphate, Monobasic, 295
Ammonium Standard Solution (1 ppm NH4), 325
Ammonium Standard Solution (2.5 ppm NH4), 325
Ammonium Standard Solution (10 ppm NH4), 325
Ammonium Sulfate, 296
Ammonium Sulfide TS, 331
Ammonium Thiocyanate, 296
Ammonium Thiocyanate TS, 331
Ammonium Vanadate, 295
Amoxicillin, 24
Ascorbic Acid, 296
Assay and Tests, General Notices, 13
Atomic Spectrometry: Emission and Absorption, 377
Atomic Weights, General Notices, 4
B
Bacillus Calmette-Guârin Vaccine, Freeze-Dried, 240
Bacterial Endotoxins, Test for, 522
Baird-Parker Agar Medium, 633
Banded Krait Antivenin, 233
Barbital, 296
Barbital Buffer pH 8.6, Mixed, 330
Barbital Sodium, 296
Barbitone, 296
Barbitone Sodium, 296
Barbituric Acid, 296
Barium Chloride, 297
Barium Chloride TS, 331
Basic Fuchsin, 308
Basic Green 4, 327
Benzene, 297
Benzene-1,3-diol, 315
Benzoic Acid, 297
Benzoyl Chloride, 297
Benzyl Alcohol, 296
Biological Assay of Antibiotics, 500
Biological Products, Determination of Aluminium, 178
Biological Products, Determination of Calcium, 178
Biological Products, Determination of Formaldehyde, 178
Biological Products, Determination of Phenol, 179
Biological Products, Determination of Thiomersal, 179
Biological Products, Glossary of Terms, 177
Biological Products, Labelling of, 177
Biological Products, Packaging and Storage for, 177
Biological Products, Preservative for, 177
Bismuth Oxynitrate, 297
Bismuth Subnitrate, 297
Bismuth Sulfite Agar Medium, 633
Blood Agar Medium, 633
Blood and Related Products, 686
Blood, Whole, 188
Blue Tetrazolium Salt, 320
Boiling Range, Determination of, 424
Boiling Temperature, Determination of, 425
Borate Buffer pH 9.0, 330
Borax, 317
Boric acid, 297
Botulinum Antitoxin, 226
Bovine Coagulation Factor Xa, 297
Brilliant Blue R, 294
Brilliant Green Agar Medium, 633
Bromelains, 297
Bromelains TS, 331
Bromine, 297
Bromine TS, 331
Bromine Water, 331
Bromocresol Blue, 326
Bromocresol Green, 326
Bromocresol Green TS, 326
Bromocresol Purple, 326
5-Bromo-2'-deoxyuridine, 297
Bromophenol Blue, 326
Bromophenol Blue TS, 331
Bromophenol Blue TS, Aqueous, 331
Bromothymol Blue, 326
Bromothymol Blue TS, 326
Buffer Solution and Media, 632
Buffer Solutions, 330
Buffered Sodium Chloride-Peptone Solution, pH 7.0, 633
Bulk density, 461
1-Butanol, 297 2-Butanone, 297
n-Butyl Alcohol, 297 tert-Butylamine, 302
Butylated Hydroxyanisole, 297
C
Cadmium Iodide, 297
Cadmium Iodide TS, 331
Caffeine, 297
Calcium, Limit Tests for, 477
Calcium Carbonate, 298
Calcium Carbonate, Chelometric Standard, 298
Calcium Chloride, 298
Calcium Chloride, Anhydrous, 298
Calcium Chloride TS, 331
Calcium Hydroxide, 298
Calcium Hydroxide TS, 331
Calcium Standard Solution (100 ppm Ca), Ethanolic, 325
Calcon, 298
Calcon Mixture, 298
Calconcarboxylic Acid, 298 Calconcarboxylic Acid Mixture, 298 dl-10-Camphorsulfonic Acid, 298
Capillary Electrophoresis, 408
Carbomer, 298
Carbon Dioxide, 298
Carbon Disulfide, 298
Carbon Tetrachloride, 298
CAS Registry Numbers, General Notices, 4
Casein, 298
Casein Digest-Soy Lecithin Polysorbate 20 Broth Medium, 633
Category, General Notices, 8
Cefaclor, 33
Cefaclor, Infrared Spectrum, 666 Cefaclor Capsules, 35
Cefaclor for Oral Suspension, 36
Cefadroxil, 36
Cefadroxil, Infrared Spectrum, 667
Cefadroxil Capsules, 38
Cefadroxil for Oral Suspension, 39
Cefazolin Sodium, 40
Cefazolin Sodium, Infrared Spectrum, 667
Cefazolin Sodium for Injection, 42
Cefotaxime Sodium, 43
Cefotaxime Sodium, Infrared Spectrum, 668
Cefotaxime Sodium for Injection, 45
Cefoxitin Sodium, 46
Cefoxitin Sodium, Infrared Spectrum, 668
Cefoxitin Sodium for Injection, 48
Ceftriaxone Sodium, 49
Ceftriaxone Sodium, Infrared Spectrum, 669
Ceftriaxone Sodium for Injection, 51
Cefuroxime Axetil, 52
Cefuroxime Axetil, Infrared Spectrum, 669
Cefuroxime Axetil Tablets, 54
Cefuroxime Sodium, 55
Cefuroxime Sodium, Infrared Spectrum, 670
Cefuroxime Sodium for Injection, 57
Cephalexin, 58
Cephalexin, Infrared Spectrum, 670
Cephalexin Capsules, 60
Cephalexin for Oral Suspension, 61
Cephalexin Tablets, 62
Cephalin TS, 331
Cerium(III) Nitrate, 298 Cerium(III) Nitrate TS, 332 Cerous Nitrate, 298
Cetostearyl Alcohol, 299
Cetrimide, 299
Cetrimide Agar Medium, 633
Charcoal, Decolorizing, 299
Chemical Name, General Notices, 4
Chloramphenicol, 63
Chloramphenicol, Infrared Spectrum, 671
Chloramphenicol Capsules, 64
Chloramphenicol Ear Drops, 65
Chloramphenicol Eye Drops, 66
Chloramphenicol Eye Ointment, 67
Chloramphenicol Sodium Succinate, 67
Chloramphenicol Sodium Succinate, Infrared Spectrum, 671
Chloramphenicol Sodium Succinate for Injection, 69
Chloride, Limit Tests for, 477
Chlorine, 299
Chlorine TS, 332
Chlorine Water, 332
Chloroacetic Acid, 299
Chloroform, 299
Chloroform Water, 299
Chloroplatinic(IV) Acid, 299
Chloroquine Phosphate, 70
Chloroquine Phosphate, Infrared Spectrum, 672
Chloroquine Phosphate Tablets, 71
Cholera Vaccine, 243
Chromatographic Separation Technique, 415
Chromic Acid Cleansing Mixture, 299
Chromotropic Acid Sodium Salt, 299
Cinchonidine, 299
Cineole, 299
Cineole, Determination of, 518
Ciprofloxacin Hydrochloride, 72
Ciprofloxacin Hydrochloride, Infrared Spectrum, 672
Ciprofloxacin Hydrochloride Tablets, 74
Citric Acid, 300
Clarity of Solution, 419
Clavulanate Potassium, 75
Cleland’s Reagent, 303
Clindamycin Hydrochloride, 78
Clindamycin Hydrochloride Capsules, 80
Clofazimine, 81
Clofazimine, Infrared Spectrum, 673
Clofazimine Capsules, 82
Clotrimazole, 83
Clotrimazole, Infrared Spectrum, 673
Clotrimazole Cream, 85
Clotrimazole Vaginal Tablets, 86
Cloxacillin Sodium, 87
Cloxacillin Sodium, Infrared Spectrum, 674
Cloxacillin Sodium Capsules, 88
Cloxacillin Sodium for Injection, 89
Cloxacillin Sodium for Oral Solution, 90
Cobalt Chloride, 300
Cobalt(II) Chloride, 300
Cobalt(II) Nitrate, 300
Cobalt(II) Nitrate Hexahydrate, 300
Cobaltous Chloride, 300
Cobra Antivenin, 232
Cobra Antivenin, Biological Assay of, 707
Cobra, King Cobra, Banded Krait and Malayan Krait Antivenin, 236
Colour Change of pH Indicators, 328
Colour of Solution, 420
Colouring Agents, General Notices, 7
Columbia Agar Medium, 633
Column Chromatography, 392
Committee, vii
Complexometric Titrations, 490
Congo Red, 326
Congo Red Paper, 326
Containers for Human Blood and Blood Components, 654
Content of Antimicrobial Agents, 509
Contents, iii
Contra-indication, General Notices, 9
Coomassie Brilliant Blue R250, 294
Copper, 300
Copper(II) Sulfate, 300
Copper(II) Sulfate TS, 332
Copper-Citric TS, 332
Cottonseed Oil, 300
o-Cresol 300
Cresol Red, 326
Cresol Red TS, 326
o-Cresolsulfonphthalein, 326
Crystal Violet, 326
Crystal Violet TS, 327
Crystallinity, 436
Cyclohexane, 300
Cyclohexane UV, 300
3-Cyclohexylpropionic Acid, 300
L-Cystine, 301
D
D-Glucose, 301
D-Glucose Monohydrate, 301
Di(2-ethylhexyl) Phthalate, 302
Diacetylmethane, 294
2,3-Diaminonaphthalene, 301
Diaveridine, 301
Diazobenzenesulfonic Acid TS, 332
Dichlorobenzene, 301
1,2-Dichloroethane, 301
2,7-Dichlorofluorescein, 301
Dichloromethane, 301
Diethylamine, 302
Diethylaminoethyldextran, 302
Diethylenediamine, 312
Dihydroquinine, 302
Dilute Hydrogen Peroxide Solution, 332
1,1-Dimethylethylamine, 302
Dimethyl Sulfoxide, 303
Dimethyl Yellow, 327
Dimethyl Yellow TS, 327
Dimethylacetamide, 302
4-Dimethylaminobenzaldehyde, 302
4-Dimethylaminocinnamaldehyde, 302
N,N-Dimethylaniline, 302, 486
Dimethylanilin, 486
Dimethylformamide, 302
2,4-Dinitrofluorobenzene, 303
Dioctyl Phthalate, 302
Diphenylamine, 303
Diphenylamine TS, 332
Diphenylamine TS1, 332
N,N’-Diphenylbenzidine, 303
Diphenylbenzidine, 303
Diphenylthiocarbazone, 303
Diphosphorus Pentoxide, 312
Diphtheria and Tetanus Vaccine, Adsorbed, 274
Diphtheria Antitoxin, 227
Diphtheria, Tetanus and Pertussis (Acellular Component) Vaccine,
Adsorbed, 276
Diphtheria, Tetanus and Pertussis Vaccine, Adsorbed, 275
Diphtheria Vaccine, Adsorbed, 245
Dipotassium Dichromate, 313
Dipotassium Dihydrogen Ethylenediaminetetraacetate, 303
Dipotassium Edetate, 303
Dipotassium Hydrogenphosphate, 303
Disintegration Test for Suppositories and Pessaries, 441
Disintegration Test for Tablets and Capsules, 442
Disodium Edetate, 303
Disodium Edetate VS, Twentieth-Molar, (0.05 M), 323
Disodium Hydrogen Orthophosphate Dihydrate, 303
Disodium Hydrogenphosphate, 303
Disodium Hydrogenphosphate, Anhydrous, 303
Disodium Hydrogenphosphate Dihydrate, 303
Disodium Tetraborate, 317
Dissolution Test, 445
Distillation Range, Determination of, 424
Dithiothreitol, 303
Dithizone, 303
Dithizone TS, 332
D-Mannose, 308
Domperidone, 94
Domperidone, Infrared Spectrum, 675
Domperidone Oral Suspension, 95
Dose, General Notices, 9
Doxycycline Hyclate, 96
Doxycycline Hyclate, Infrared Spectrum, 675
Doxycycline Hyclate Capsules, 98
Dried Factor IX Fraction, 203
Dried Factor VII Fraction, 198
Dried Factor VIII (rDNA), 201
Dried Factor VIII Fraction, 200 Dried Prothrombin Complex, 205
E
Edetic Acid, 303
Electrophoresis, 399
Enterobacteria Enrichment Broth-Mossel Medium, 634
Eosin Y, 303
Eosin Y TS, 332
Eosin Yellowish Y, 303
Epsom Salts, 308
Eriochrome Black T, 310
Erythromycin, 99
Erythromycin, Infrared Spectrum, 676
Erythromycin Delayed-release Tablets, 102
Erythromycin Ethylsuccinate, 103
Erythromycin Ethylsuccinate, Infrared Spectrum, 676
Erythromycin Ethylsuccinate for Oral Suspension, 105
Erythromycin Ethylsuccinate Tablets, 106
Erythromycin Stearate, 107
Erythromycin Stearate, Infrared Spectrum, 677
Erythromycin Stearate Tablets, 109
Esters, Determination of, 483
Ester Value, 480
Estimation of Body Surface Area, 368
Estimation of Lean Body Weight, 371
Ethanol, 303
Ethanol, Absolute, 303
Ethanol, Aldehyde-free, 304
Ethanol, Determination of, 494
Ethanol, Diluted, 304
Ethanol, Neutralized, 304
Ether, 304
Ether, Peroxide-free, 304
Ethyl Acetate, 304
2-Ethylhexanoic Acid, 304
2-Ethylhexoic Acid, 304 Ethyl Methyl Ketone, 297
Ethylbenzene, 304
Ethylene Chloride, 301
Ethylene Glycol Monomethyl Ether, 309
Ethylene Oxide, 304
Ethylenediaminetetra-acetic Acid, 303
Eucalyptol, 299
Extractives, 519
F
Factor VIII, Cryoprecipitated, 203
Ferric Ammonium Sulfate, 295
Ferric Chloride, 307
Fibrin Sealant Kit, 207
Flowability, 461
Fluid Mercapto Acetate Medium, 615 Fluid Thioglycolate Medium, 615
Fluorescence Spectrophotometry, 378
Fluoride, Limit Tests for, 477
Fluorine Standard Solution (400 ppm F), 325
1-Fluoro-2,4-dinitrobenzene, 303
Foreign Matter, 516
Formaldehyde Solution, 304
Formalin, 304
Formamide, 304
Formic Acid, 305
Formic Acid, Anhydrous, 305
Freezing Temperature, Determination of, 423
Fresh Frozen Plasma, 192
Freshly and Recently Prepared, General Notices, 6
Friability of Uncoated Tablets, 460
Fructose, 305 Fuchsin TS, Decolorized, 332
G
Gas Chromatography, 394
Gelatin, 305
General Identification Tests, 470
General Information for Biological Products, 177
General Notices, 1
General Testing Methods for Biological Products, 178
Gentamicin Sulfate, 111
Gentamicin Sulfate, Infrared Spectrum, 677
Gentamicin Sulfate Cream, 113
Gentamicin Sulfate Eye Drops, 114
Gentamicin Sulfate Injection, 114
Gentamicin Sulfate Ointment, 115
Glass Containers, 642
Glutaraldehyde, 305
Glycerin, 305
Glycerol, 305
Glycerol (85 Per Cent), 305
Glyoxal Bis(2-hydroxyanil), 305
Glyoxaline, 306
Graphic Formulae, General Notices, 4
Green Pit Viper Antivenin, 234
Green Pit Viper, Malayan Pit Viper, and Russell’s Viper Antivenin, 235
Griseofulvin, 115
Griseofulvin, Infrared Spectrum, 678
Griseofulvin Tablets, 116
Guaiacol, 305
Guaiacol TS, 332 Guanine, 305
H
Haemophilus Type B Conjugate Vaccine, 245
Heavy Metals, Limit Tests for, 474
Helianthin, 327
Helium, 305
Hematotoxic Polyvalent Antivenin, 235
Hemoglobin Concentration by Hemiglobincyanide Method, Determination of, 687
Heparin in Coagulation Factors, Biological Assay of, 686
Hepatitis A Vaccine, Inactivated, 247
Hepatitis B Immunoglobulin, 215 Hepatitis B Vaccine, Recombinant, 248 n-Heptane, 305
1-Heptanesulfonic Acid Sodium Salt, 316
Hexachloroplatinic(IV) Acid Hexahydrate, 299
Hexadimethrine Bromide, 305
Hexamine, 309
High-pressure Liquid Chromatography, 396
Holmium Oxide, 306
House Dust Mite Allergen Vaccine, 250 Human Blood and Blood Products, 692
Human Coagulation Factor II, Biological Assay of, 701
Human Coagulation Factor IX, Biological Assay of, 697
Human Coagulation Factor VII, Biological Assay of, 701
Human Coagulation Factor VIII, Biological Assay of, 696
Human Coagulation Factor X, Biological Assay of, 697
Human Normal Immunoglobulin for Intravenous Administration, 211
Human Thrombin, 321
Human von Willebrand Factor, Biological Assay of, 702
Hydrazine Sulfate, 306
Hydrazinium Sulfate, 306
Hydriodic Acid, 306
Hydrochloric Acid, 306
Hydrochloric Acid, Dilute, 306
Hydrochloric Acid, 0.1 M Methanolic, 306
Hydrochloric Acid VS, Molar, (1 M), 323
Hydrocortisone Acetate, 117
Hydrocortisone Acetate, Infrared Spectrum, 678
Hydrocortisone Acetate and Neomycin Sulfate Eye Ointment, 119
Hydrogen Peroxide Solution, Strong, 306
Hydrogen Peroxide TS (10 volumes), 332
Hydrogen Peroxide TS (20 volumes), 332
Hydrogen Peroxide TS (100 volumes), 332
Hydrogen Sulfide, 306
Hydrogen Sulfide TS, 332
Hydroquinine, 302
Hydroxy Naphthol Blue, 306
Hydroxyl Value, 482
Hydroxylamine Hydrochloride, 306
Hydroxylamine in Ethanol (60 per cent) TS, 332
Hydroxylamine in Ethanol (90 per cent) TS, 332
Hydroxylamine TS, 332
Hydroxylammonium Chloride, 306
5-Hydroxymethylfurfural, 306
8-Hydroxyquinoline, 306 Hypophosphorous TS, 332
I
Identification, General Notices, 12
Idoxuridine, 120
Idoxuridine Eye Drops, 121
Imidazole, 306
Imidazole Buffer Solution pH 7.3, 330
Immunochemical Methods, 688
Immunoglobulin, 209
Impurities, General Notices, 13
Indane-1,2,3-trione, 310
Indigo Carmine, 327
Indigo Carmine TS, 327
Indigotindisulfonate, 327
Indophenol Blue, 306
Influenza Vaccine, Inactivated (Split Virion), 253
Influenza Vaccine, Inactivated (Surface Antigen), 254
Influenza Vaccine, Inactivated (Surface Antigen, Virosome), 255
Influenza Vaccine, Inactivated (Whole Virion), 252
Infrared Reference Spectra, 664
Infrared Spectrophotometry, 372
Insoluble Matter, Determination of, 436
Introduction, xxiii
Iodine, 306
Iodine and Potassium Iodide TS, 332
Iodine Bromide, 306
Iodine Bromide TS, 332
Iodine Chloride TS, 332
Iodine Monobromide, 306
Iodine Monochloride, 307
Iodine TS, 332
Iodine Value, 480
Iodine VS, Twentieth-Molar, (0.05 M), 323
Iodobromide TS, 332
Iodochloride TS, 332
5-Iodouracil, 307
Iron, Limit Tests for, 478
Iron Standard Solution (10 ppm Fe), 325
Iron(III) Chloride, 307
Iron(III) Chloride TS, 332
Iron(III) Chloride-Sulfamic Acid TS, 332
Iron(III) Sulfate, 307
Isoamyl Alcohol, 296
Isobutyl Alcohol, 309
Iso-octane, 321
Isopentyl Alcohol, 296
Isopropanol, 312
Isopropyl Myristate, 307 Isopropyl Tetradecanoate, 307
J
Japanese Encephalitis Vaccine, Inactivated, 256
K
Kaolin, Light, 307 King Cobra Antivenin, 232
L
Labelling, General Notices, 12
Lactose, 307
Lactose Broth Medium, 634
Lanthanum Nitrate, 307
Lead, Limit Tests for, 478
Lead Acetate TS, 332
Lead Dioxide, 308
Lead Nitrate Stock Solution, (100 ppm Pb), 326
Lead Nitrate VS, Twentieth-Molar, (0.05 M), 323
Lead Standard Solution (1 ppm Pb), 326
Lead Standard Solution (2 ppm Pb), 326
Lead Standard Solution (10 ppm Pb), 326
Lead(II) Acetate, 307
Lead(II) Nitrate, 308
Lead(IV) Oxide, 308
Levine Eosin-Methylene Blue Agar Medium, 634 Levulose, 305
Light Petroleum, 311
Limit of Content, General Notices, 6
Limit Tests, 474
Lincomycin Hydrochloride, 123
Lincomycin Hydrochloride, Infrared Spectrum, 679
Lincomycin Hydrochloride Injection, 124
Lithium Hydroxide, 308
Litmus, 327
Litmus Paper, 327
Litmus Paper, Blue, 327
Litmus Paper, Red, 327
Litmus TS, 327
Loss on Drying, 437
Loss on Ignition, 437
M
MacConkey Agar Medium, 634
MacConkey Broth Medium, 634
Macrogol 400, 312
Magenta, Basic, 308
Magenta TS, Decolorized, 332
Magnesium Oxide, 308
Magnesium Sulfate, 308
Magnesium Sulfate TS, 333
Malachite Green, 327
Malachite Green TS, 327
Malayan Krait Antivenin, 233
Malayan Pit Viper Antivenin, 234
Manganese Dioxide, 308
Manganese(II) Sulfate, 308
Manganese(IV) Oxide, 308
Mannitol-Salt Agar Medium, 634
Mannose, 308
Mass Spectrometry, 382
Materials for Chromatography, 335
Mayer’s Reagent, 333
Measles, Mumps and Rubella Vaccine, Live, 271
Measles Vaccine, Live, 258
Medicine Dropper, 343
Melting Range, Determination of, 421
Melting Temperature, Determination of, 421
Meningococcal Polysaccharide Vaccine, 259
Mercaptoacetic Acid, 320
2-Mercaptoethanol, 308
Mercuric Oxide, 309
Mercuric-Potassium Iodide TS, 333
Mercuric-Potassium Iodide TS, Alkaline, 333
Mercurous Nitrate Dihydrate, 308
Mercury(I) Nitrate, 308
Mercury(I) Nitrate TS, 333
Mercury(II) Chloride, 308
Mercury(II) Iodide, 308
Mercury(II) Oxide, Yellow, 309
Mercury(II) Sulfate TS, 333
Metacresol Purple, 327
Metacresol Purple TS, 327
Metalphthalein, 309
Metanil Yellow, 327
Metanil Yellow TS, 327
Methanesulfonic Acid, 309
Methanol, 309
Methanol, Aldehyde-free, 309
Methanol, Anhydrous, 309
Methanol, Determination of, 485
Methenamine, 309
4-Methoxybenzaldehyde, 296
2-Methoxyethanol, 309
2-Methoxyphenol, 305
Methoxyl, Determination of, 498
Methyl Alcohol, 309
Methyl Isobutyl Ketone, 309
Methyl Orange, 327
Methyl Orange TS, 329
Methyl Orange-Xylene Cyanol FF TS, 329
3-Methyl-2-benzothiazolinone Hydrazone Hydrochloride Hydrate, 309
3-Methyl-1-Butanol, 296
4-Methyl-2-Pentanone, 309
2-Methyl-1-Propanol, 309
Methyl Purple TS, 329
Methyl Red, 329
Methyl Red Sodium Salt, 309
Methyl Red TS, 329
Methyl Red-Methylene Blue TS, 329
Methylbenzene, 321
Methylcellulose 450, 309
N-Methylformamide, 310
Methylene Blue, 310
Methylene Blue TS, 327
Methylene Chloride, 301
N,N’-Methylenebisacrylamide, 310
2-Methylphenol, 300
Metronidazole, 125
Metronidazole, Infrared Spectrum, 679
Metronidazole Tablets, 126
Miconazole Nitrate, 127
Miconazole Nitrate, Infrared Spectrum, 680
Miconazole Nitrate Cream, 128
Microbial Contamination, Limits for, 637
Microbial Enumeration Tests, 622
Microbial Limit Tests, 621
Microbiological Attributes of Non-sterile Pharmaceutical Products, 637
Minimum Fill, 450
Molecular Formulae, General Notices, 4
Molecular Weights, General Notices, 4
Mordant Black 11, 310
Mordant Black 11 Mixture, 310
Mordant Black 17, 298
Mordant Black 17 Mixture, 298
Mordant Red 3, 326 O
Mumps Vaccine, Live, 261
N
Nalidixic Acid, 129
Nalidixic Acid, Infrared Spectrum, 680
Nalidixic Acid Tablets, 130
Names, Symbols and Atomic Weights of Elements, 341
Naphthalene, 310
Naphthalene Black 12B, 310
1,3-Naphthalenediol, 310
Naphtharson, 320
α -Naphthol, 310
β -Naphthol, 310
1-Naphthol, 310
2-Naphthol, 310
1-Naphthol TS, Dilute, 333
2-Naphthol TS, 333
p-Naphtholbenzein, 329
1-Naphtholbenzein, 329
1-Naphtholbenzein TS, 329
Naphthoresorcinol, 310
N-(1-Naphthyl)ethylenediamine Dihydrochloride, 310
Neomycin Sulfate, 131
Neomycin Sulfate, Infrared Spectrum, 681
Neomycin Sulfate Tablets, 132
Nessler's Reagent, 333,
Neurotoxic Polyvalent Antivenin, 236
Neutral Red, 329
Neutral Red TS, 329
Ninhydrin, 310
Ninhydrin TS, 333
Nitric Acid, 310
Nitric Acid, Dilute, 311
Nitrogen, 311
Nitrogen, Determination of, 499
Nitrogen for Chromatography, 311
Nitromethane, 311
Nitro-vanado-molybdic TS, 333
Non-aqueous Titration, 488
Norfloxacin, 133
Norfloxacin, Infrared Spectrum, 681
Norfloxacin Tablets, 134
Nuclear Magnetic Resonance Spectrometry, 385
Nucleic Acid Amplification, 690
Nystatin, 136
Nystatin, Infrared Spectrum, 682
Nystatin Oral Suspension, 136
Nystatin Tablets, 137
O
Octoxinol 10, 311
Octoxynol 10, 311
Official, General Notices, 3
Official Name, General Notices, 3
Official Standards, General Notices, 3
Optical Rotation, Determination of, 425
Ordinary Impurities, 486
Orthophosphoric Acid, 312
Osmolality, 465
Oxalic Acid, 311
Oxalic Acid TS, 333
Oxirane, 304 Oxygen, 311
Oxygen Flask Combustion, 489
Oxytetracycline Hydrochloride, 138
Oxytetracycline Hydrochloride, Infrared Spectrum, 682
Oxytetracycline Hydrochloride Capsules, 139
P
Package and Storage, General Notices, 10
PAN, 314
Papain, 311
Paper Chromatography, 391
Particulate Matter in Injections, 451
Patton and Reeder’s Reagent, 298
Penicillin V Potassium, 140
Penicillin V Potassium, Infrared Spectrum, 683
Penicillin V Potassium for Oral Solution, 142
Penicillin V Potassium Tablets, 142
Pentane, 311 n-Pentane, 311
1-Pentanesulfonic Acid Sodium Salt, 317
Pepsin, 311 Perchloric Acid, 311
Perchloric Acid VS, Tenth-Molar, (0.1 M), 323
Percutaneous Bacillus Calmette-Guârin Vaccine, 242
Peroxide Value, 483
Pertussis Vaccine, Adsorbed, 262
Pertussis Vaccine (Acellular Component), Adsorbed, 263 Petroleum Ether, 311 pH, Determination of, 432 pH Indicators, 326 Pharmaceutical Dosage Forms, 343
Phenol Red, 329
Phenol Red TS, 329
Phenol, 311
Phenolphthalein, 329
Phenolphthalein TS, 329
Phenolphthalein TS, Dilute, 329
2-Phenoxyethanol, 312
Phenylazothioformic Acid 2-Phenylhydrazone, 303
Phosphate Buffer pH 6.8, Mixed, 330
Phosphate Buffer pH 7.0, Mixed, 0.067 M, 330
Phosphate Buffer pH 7.2, 633
Phosphate Buffers, 330
Phospholipid, 312
Phosphomolybdic Acid, 312
Phosphoric Acid, 312
Phosphorus Pentoxide Desiccant, 312
Phthalein Purple, 309
Picric Acid, 321
Piperazine Hexahydrate, 312
Piperazine Hydrate, 312
Plasma, Platelet-poor, 312
Plasma for Fractionation, 193
Plasma Substrate 2, 312
Plastic Containers, 645
Platelet Concentrate, 192
Platelet Substitute, 312
Platinic Chloride, 299
Pneumococcal Polysaccharide Conjugate Vaccine, Adsorbed, 265
Pneumococcal Polysaccharide Vaccine, 265
Polarography, 512
Poliomyelitis Vaccine, Inactivated, 267
Poliomyelitis Vaccine, Oral, 266
Polyethyleneglycol 400, 312
Polymethylphenylsiloxane, 313
Polyoxyethylene(20) Sorbitan Monolaurate, 313
Polyoxyethylene(20) Sorbitan Monooleate, 313
Polysorbate 20, 313
Polysorbate 80, 313
Polystyrene Film, Infrared Spectrum, 664
Polyvinyl Alcohol, 313
Potash Alum, 295
Potassium Bromide, 313
Potassium Carbonate, Anhydrous, 313
Potassium Chloride, 313
Potassium Chromate, 313
Potassium Chromate TS, 333
Potassium Cyanide, 313
Potassium Dichromate, 313
Potassium Dichromate TS, 333
Potassium Dihydrogen Orthophosphate, 313
Potassium Dihydrogencitrate, 313
Potassium Dihydrogenphosphate, 313
Potassium Ferricyanide, 314 Potassium Ferrocyanide, 314
Potassium Hexacyanoferrate(II), 314
Potassium Hexacyanoferrate(III), 314
Potassium Hexacyanoferrate(II) TS, 333 Potassium Hexacyanoferrate(III) TS, 333
Potassium Hydrogentartrate, 314
Potassium Hydroxide, 314
Potassium Hydroxide, 0.5 M Ethanolic, 314
Potassium Hydroxide TS, Ethanolic, 333
Potassium Hydroxide VS, Ethanolic, Half-Molar, (0.5 M), 324
Potassium Hydroxide VS, Molar, (1 M), 323
Potassium Hydroxide VS, Tenth-Molar, (0.1 M), 323
Potassium Iodide, 314
Potassium Iodide and Starch Solution, 333
Potassium Iodide TS, 333
Potassium Iodobismuthate TS, 333
Potassium Iodobismuthate TS, Acetic, 333
Potassium Iodobismuthate TS, Dilute, 333
Potassium Iodoplatinate TS, 333
Potassium Nitrate, 314
Potassium Permanganate, 314
Potassium Permanganate and Phosphoric Acid TS, 334
Potassium Permanganate TS, 333
Potassium Permanganate VS, Fiftieth-Molar, (0.02 M), 324
Potassium Phosphate, Dibasic, 303
Potassium Sodium Tartrate, 314
Potassium Sulfate, 314
Potassium Tetroxalate, 314
Potassium Trihydrogen Dioxalate, 314
Potato Dextrose Agar Medium, 634
Potentiometric Determination, 492
Potentiometric Titration, 492
Potentiometry, 491
Poured density, 461
Powder Fineness and Sieves, 339
Powder Flow, 462
Preface, v
Prekallikrein Activator, Test for, 686
Printing Types, General Notices, 4
Propanal, 314
Propane-1,2-diol, 314
1-Propanol, 314
2-Propanol, 312
2-Propanol, Determination of, 485
Propionaldehyde, 314 n-Propyl Alcohol, 314 Propylene Glycol, 314
Protamine Sulfate, 312
Pseudomonas Agar for Detection of Fluorescin Medium, 634
Pseudomonas Agar for Detection of Pyocyanin Medium, 635
Purified Water, 143
Purified Water, Sterile, 143
Pyrazinamide, 144
Pyrazinamide, Infrared Spectrum, 683
Pyrazinamide Tablets, 145
Pyridine, 315
Pyridine, Anhydrous, 315
1-(2-Pyridylazo)-2-naphthol, 314
Pyridylazonaphthol, 314
Pyridylazonaphthol TS, 334 Pyrogen Test, 521
Q
Quinine Sulfate, 146, 315
Quinine Sulfate Tablets, 148
8-Quinolinol, 306
R
Rabies Antiserum, 230
Rabies Antiserum, Fluorescein-conjugated, 315
Rabies Immunoglobulin, 216
Rabies Vaccine, Inactivated, 269
Raman Spectrometry, 380
Rappaport-Vassiliadis Broth Medium, 635
Reagents, 294
Red Cell Concentrate, 189
Reference Substances, 336
Refractive Index, Determination of, 425
Reinforced Medium for Clostridia Medium, 635
Related Foreign Steroids, 390
Related Impurities in Phenothiazines, 390
Related Substances in Sulfonamides, 390
Relationship Between Reaction of Solution, Approximate pH and
Colour of Certain Indicators, 484
Relative Density, Determination of, 428
Resazurin (Sodium), 329
Residual Titrations, 509
Residue on Evaporation of Volatile Oils, 519
Resistance to Crushing of Tablets, 460
Resorcinol, 315
Resorcinol with Copper(II) Sulfate TS, 334
Rh Group of Donors, Determination of, 694
Rubella Vaccine, Live, 271
Russell’s Viper Antivenin, 235
S
Sabouraud Dextrose Agar Medium, 635
Sabouraud Dextrose Agar with Antibiotics Medium, 635
Sabouraud Dextrose Broth Medium, 635
Safety of Drugs, General Notices, 8
Salicylic Acid, 315
Saline pH 7.4, Phosphate-buffered, 331
Saline TS, 334
Saline TS, Pyrogen-free, 334
Sampling, 516
Saponification Value, 481
Selenium, 315
Selenium, Limit Tests for, 479
Selenium Standard Solution (1 ppm Se), 326
Sesame Oil, 315
Sets for the Transfusion of Human Blood and Blood Components, 656
O-Sialic Acid, 294
Significant Figures and Tolerances, General Notices, 5
Silica Gel, Self-Indicating, 315
Silver Diethyldithiocarbamate, 315
Silver Diethyldithiocarbamate TS, 334
Silver Nitrate, 315
Silver Nitrate TS, 334
Silver Nitrate VS, Tenth-Molar, (0.1 M), 324
Silver Oxide, 315
Simulated Gastric Fluid TS, 334
Simulated Intestinal Fluid TS, 334
Sinapic Acid, 316
Sinapinic Acid, 316
Size-exclusion Chromatography, 398
Sodium 1-Heptanesulfonate, 316
Sodium 1-Pentanesulfonate, 317
Sodium Acetate, 316
Sodium Azide, 316
Sodium Bicarbonate, 316
Sodium Carbonate, 316
Sodium Carbonate, Anhydrous, 316
Sodium Chloride, 316
Sodium Citrate, 316
Sodium Citrate Dihydrate, 316
Sodium Diethyldithiocarbamate, 316
Sodium Dihydrogen Orthophosphate Monohydrate, 316
Sodium Dihydrogenphosphate, 316
Sodium Dihydrogenphosphate Monohydrate, 316
Sodium Dithionite, 316
Sodium Dodecyl Sulfate, 316
Sodium Fluoride, 316
Sodium Hexanitrocobaltate(III) TS, 334
Sodium Hydrogencarbonate, 316
Sodium Hydrosulfite, 316
Sodium Hydroxide, 316
Sodium Hydroxide TS, 334
Sodium Hydroxide, Ethanolic, 316
Sodium Hydroxide VS, Ethanolic, Tenth-Molar, (0.1 M), 324
Sodium Hydroxide VS, Molar, (1 M), 324
Sodium Hypochlorite Solution, 317
Sodium Hypochlorite TS, 334
Sodium Hypochlorite TS, Dilute, 334
Sodium Hypophosphite, 317
Sodium Lauryl Sulfate, 316
Sodium Mercaptoacetate, 318
Sodium Metabisulfite, 317
Sodium Metabisulfide TS, 334
Sodium Molybdate, 317
Sodium Molybdotungstophosphate TS, 334
Sodium Nitrite, 317
Sodium Nitrite TS, 334
Sodium Nitroferricyanide, 317
Sodium Nitroprusside, 317
Sodium Oxalate, 317
Sodium Perchlorate, 317
Sodium Phosphate-Sodium Chloride Buffer TS, 334
Sodium Phosphinate Monohydrate, 317
Sodium Pyrosulfite, 317
Sodium Sulfate, Anhydrous, 317
Sodium Sulfide, 317
Sodium Sulfide TS, 334
Sodium Sulfide TS1, 334 Sodium Tartrate, 317
Sodium Tetraborate, 317
Sodium Tetradeuteriodimethylsilapentanoate, 318
Sodium Tetraphenylborate, 318
Sodium Tetraphenylborate TS, 334
Sodium Thioglycolate, 318
Sodium Thiosulfate, 318
Sodium Thiosulfate VS, Tenth-Molar, (0.1 M), 324
Sodium Tungstate, 318
Solidification Temperature of Fatty Acids, 483
Solochrome Black, 310
Solochrome Dark Blue, 298
Solochrome Dark Blue Mixture, 298
Solubility, General Notices, 7
Soybean-Casein Digest Agar Medium, 635
Soybean-Casein Digest Broth Medium, 635
Soybean-Casein Digest Medium, 616
Specific Gravity, Determination of, 427
Specific Rotation, Determination of, 425
Standard Solution, 325
Standard Solutions, 325
Stannous Chloride, 321
Starch, Corn, 318
Starch, Potato, 318
Starch, Rice, 318
Starch, Soluble, 318
Starch Iodide Paper, 319
Starch TS, 334
Starches, 318
Statistical Analysis of Results of Biological Assays and Tests, 528
Sterility Test, 615
Sterilization and Sterility Assurance, 658
Steroids, Identification of, 389
Stock buffer solution, 633
Strength(s) Available, General Notices, 8
Streptomycin Sulfate, 149
Streptomycin Sulfate Injection, 151
Strychnine Sulfate, 319
Sucrose, 319
Sulfamethoxazole, 152
Sulfamethoxazole, Infrared Spectrum, 684
Sulfamethoxazole and Trimethoprim Oral Suspension, 153
Sulfamethoxazole and Trimethoprim Tablets, 155
Sulfamic Acid, 319
Sulfanilamide 319
Sulfate, Limit Tests for, 479
Sulfated Ash, Determination of, 480
Sulfathiazole, 319
Sulfur Dioxide, 319
Sulfuric Acid, 319
Sulfuric Acid, Dilute, 319
Sulfuric Acid, Ethanolic, 319
Sulfuric Acid, Nitrogen-free, 319
Sulfuric Acid VS, Half-Molar, (0.5 M), 325
Sulfuric Acid VS, Twentieth-Molar, (0.05 M), 325
Sulfuric Acid-Formaldehyde TS, 334 Swelling Index, 519
T
Talc, 319
Tapped density, 461
Tartaric Acid, 320
Test for Specified Micro-organisms, 627
Test Solutions, 331
Tetanus and Diphtheria Vaccine for Adult Use, Adsorbed, 278
Tetanus Antitoxin, 229
Tetanus Immunoglobulin, 219
Tetanus Vaccine, Adsorbed, 273
Tetrabutylammonium Hydroxide VS, Tenth-Molar, (0.1 M), 325
Tetrabutylammonium Iodide, 320
Tetracycline Hydrochloride, 157
Tetracycline Hydrochloride, Infrared Spectrum, 684
Tetracycline Hydrochloride Capsules, 158 Tetracycline Hydrochloride Tablets, 159 n-Tetradecane, 320 Tetradecylammonium Bromide, 320
Tetraheptylammonium Bromide, 320
Tetrahydrofuran, 320
Tetramethylene Oxide, 320
N,N,N’,N’-Tetramethylethylenediamine, 320
Tetramethylsilane, 320
Tetrathionate Bile Brilliant Green Broth Medium, 635
Tetrazolium Blue, 320
Tetrazolium Blue TS, Alkaline, 334
Thiazole Yellow, 329
Thin-layer Chromatography, 387
Thioacetamide, 320
Thioactamide Reagent, 320
Thioglycolic Acid, 320
Thiourea, 320
Thorin, 320
Thrombin, 321
Thrombin Bovine, 321
Thymol, 321
Thymol Blue, 329
Thymolphthalein, 329
Thymolphthalein TS, 329 Thymolsulfonphthalein, 329
Tin(II) Chloride, 321
Tin(II) Chloride TS, Stronger Acid, 334
Titan Yellow, 329
Titan Yellow Paper, 330
Titan Yellow TS, 330
Title, General Notices, 3
Tobramycin, 160
Tobramycin Sulfate, 162
Tobramycin Sulfate Injection, 163
Tolperisone Hydrochloride, 164
Tolperisone Hydrochloride, Infrared Spectrum, 685
Tolperisone Hydrochloride Tablets, 165 Toluene, 321 p-Toluidine, 321 Total Ash, 518
Total Organic Carbon, 466
Total Solids, 520
Toxoids, Bacterial, 239
Trichloroacetic Acid, 321
Triethylamine, 321
Triketohydrindene Hydrate TS, 333
Trimethoprim, 166
Trimethoprim, Infrared Spectrum, 685 2,2,4-Trimethylpentane, 321
Trimethylpentane, 321
2,4,6-Trinitrophenol, 321
Trinitrophenol TS, 334
Triple Sugar-Iron-Agar Medium, 635
Tris(hydroxymethyl) aminomethane, 321
Tris(hydroxymethyl) methylamine, 321
Tris-chloride Buffer pH 7.5, 331
Tris-EDTA BSA Buffer Solution pH 8.4, 331
Trisodium Citrate Dihydrate, 316
Trometamol, 321
Tromethamine, 321
Tropaeolin D, 327 Tropaeolin O, 330
Tropaeolin OO, 330
Tropaeolin OO TS, 330
Tuberculin Purified Protein Derivative, 287
Turbidimetry and Nephelometry, 379
Typhoid Polysaccharide Vaccine, 279 Typhoid Vaccine, Oral, 280
U
Ultraviolet and Visible Spectrophotometry, 374
Uniformity of Dosage Units, 454
Units of Potency of Antibiotics, General Notices, 6
Unsaponifiable Matter, 481 Urea, 321
V
Vaccines, 239, 708
Vaccines, Abnormal Toxicity Test for, 234
Vaccines, Allergen, 239
Vaccines, Aluminium, Determination of, 239
Vaccines, Bacterial, 239
Vaccines, Calcium, Determination of, 239
Vaccines, Combined, 239
Vaccines, Formaldehyde, Determination of, 234
Vaccines, Freeze-dried, Water of, 234
Vaccines, Packaging and Storage, 239
Vaccines, Phenol, Determination of, 239
Vaccines, Sterility Test for, 234
Vaccines, Thiomersal, Determination of, 239
Vaccines, Viral, 239
Vancomycin Hydrochloride, 167
Vancomycin Hydrochloride for Injection, 169
Varicella Immunoglobulin, 220
Varicella Vaccine, Live, 281
Violet Red Bile Dextrose Agar Medium, 636
Viscosity, Determination of, 428
Vogel-Johnson Agar Medium, 636
Volatile Oil, Determination of, 516
Volumetric Apparatus, 337
Volumetric Solution, 323 Volumetric Solutions, 322
W
Warning and Precaution, General Notices, 9
Water, 322
Water, Carbon Dioxide-free, 322
Water, Determination of, 433
Water, Particle-free, 322
Water and Loss on Drying, General Notices, 13
Water Conductivity, 467
Water for Hemodialysis, 173
Water for Inhalation, Sterile, 172
Water for Injection, 170
Water for Injection, Bacteriostatic, 171
Water for Injection, Sterile, 172
Water for Irrigation, Sterile, 172
Water-soluble Ash, 519
Weight per Millilitre, Determination of, 427
Weights and Balances, 338
Weights and Measures: SI Units, 342
X
Xanthydrol, 322
X-ray Diffraction, 437
X-Ray Fluorescence Spectrometry, 381
Xylene Cyanol FF, 322
Xylene Cyanole FF, 322
Xylenol Orange, 322
Xylenol Orange Mixture, 322 Xylose-Lysine-Deoxycholate Agar Medium, 636
Y
Yellow Fever Vaccine, 281
Z
Zinc, 322
Zinc, Granulated, 322
Zinc Powder, 322
Zinc Sulfate VS, Twentieth-Molar, (0.05 M), 325
Periodic Table of the Elements
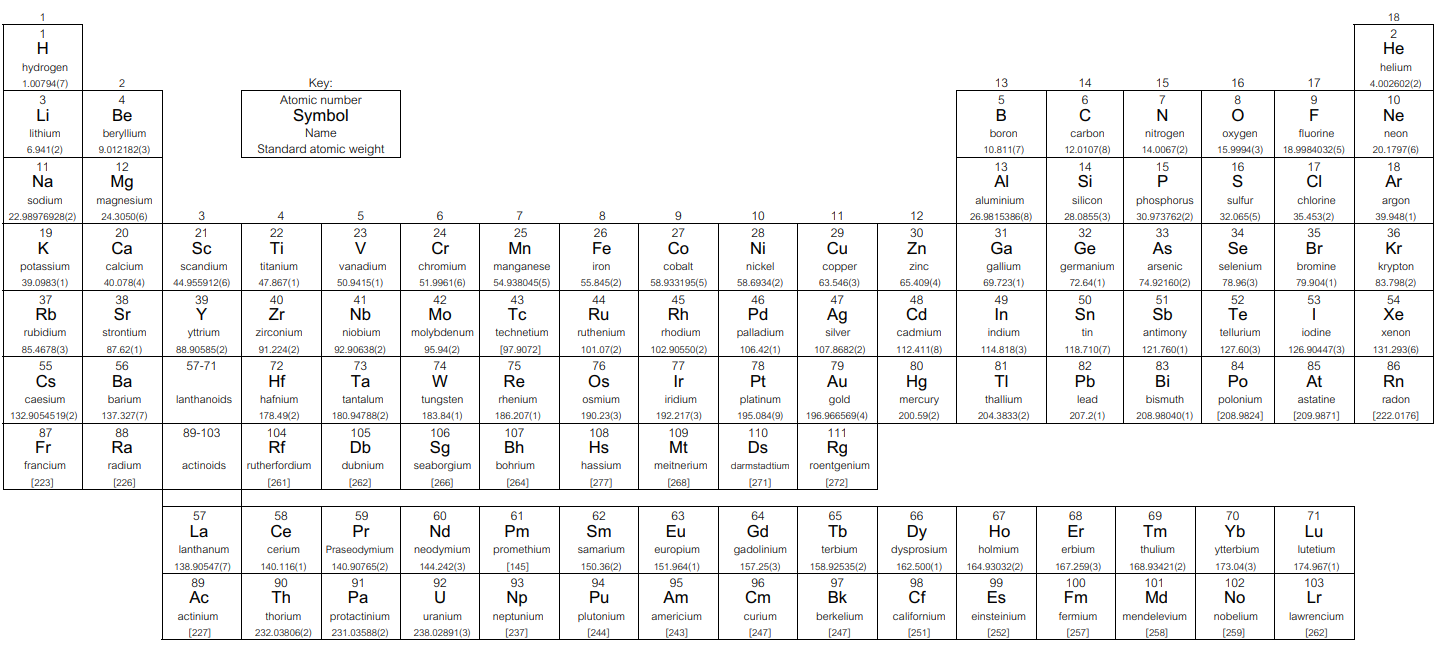
Notes
1. "Aluminum" and "cesium" are commonly used alternative spellings for "aluminium" and "caesium". For elements that have no stable or long-lived nuclides, the mass number of the nuclide with the longest confirmed half-life is listed between square brackets.
2. IUPAC 2005 standard atomic weights are listed with uncertainties in the last figure in parentheses (M. E. Wieser, "Atomic weights of the elements 2005 (IUPAC Technical Report)," Pure Appl. Chem., 78(11), 2006, pp. 2054-2056.).