ตำรายาของประเทศไทย
Thai Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health
Apparatus
The apparatus typically consists of the following:
(a) a digestion flasks, polytetrafluoroethylene, perfluoroalkoxy polymer, quartz or glass flasks with a volume of 20 to 150 mL, fitted with a tightly closed closure, a valve to adjust the pressure inside the container and a polytetrafluoroethylene tube to allow release of gas;
(b) a programmable microwave oven (e.g., with a magnetron frequency of 2450 MHz, with a selectable output from 0 to 1500 ± 70 W in 1 per cent increments);
(c) an atomic spectrometer, an inductively coupled plasma-atomic emission spectrometer, or an inductively coupled plasma-mass spectrometer.
Method
Carry out the test as described in the “Atomic Spectrometry: Emission and Absorption” (AAS) (Appendix 2.3), “Inductively Coupled Plasma-Atomic Emission Spectrometry” (ICP-AES) (Appendix 2.10), or “Inductively Coupled Plasma-Mass Spectrometry” (ICP-MS) (Appendix 2.10). Deviations from the experimental parameters of the sample preparation procedure and the method description below are acceptable provided that the validation requirements are met and the system suitability test is fulfilled on the day of the analysis.
SAMPLE PREPARATION
(Note Clean all the glassware and laboratory equipment with a 1 per cent w/v solution of nitric acid before use. All liquid samples should be weighed.)
Use either of the following methods.
Method I Wet Digestion
FOR ARSENIC, CADMIUM AND LEAD
Test solution Transfer about 250 mg of the herbal drug, coarsely powdered and accurately weighed, to a 100-mL glass beaker (in case of liquid samples, use 5 g). Add 2.5 mL of a 50 per cent v/v solution of heavy metal-free nitric acid, mix well, cover, and heat on a water-bath for 15 minutes. Allow to cool and add 5 mL of heavy metal-free nitric acid. Reheat on a water-bath for 60 minutes or until completely digested. Transfer the solution to a 50-mL volumetric flask and dilute to volume with water. Filter or centrifuge at 3421 × g (3000 rpm) for 10 minutes, if necessary. Prepare simultaneously the blank solution by carrying out the digestion in the same manner as for the test solution.
FOR MERCURY
Proceed as directed under the determination for arsenic, cadmium and lead except using a 100-mL volumetric flask in place of a 100-mL beaker and heat in a water-bath. The temperature of a water-bath is 60°.
Method II Closed Vessel Digestion or Microwave Digestion
TEST SOLUTION In a digestion flask, place the prescribed quantity of the substance to be examined (about 500 mg of the herbal drug, in No. 1400 powder). Add 4 mL of heavy metal-free hydrochloric acid and 6 mL of heavy metal-free nitric acid. Close the flask tightly.
Place the digestion flasks in the microwave oven. Carry out the digestion in 3 steps according to the following programme, used for 7 flasks each containing the test solution: 80 per cent power for 15 minutes, 100 per cent power for 5 minutes, 80 per cent power for 20 minutes.
At the end of the cycle, allow the flasks to cool in air or water. After cooling, open each digestion flask and introduce the clear, colourless solution obtained into a 50-mL volumetric flask. Rinse each digestion flask with two 15-mL portions, of heavy metal-free dilute nitric acid, collect the rinsings in the volumetric flask and dilute to 50.0 mL with water. Modifiers (e.g., in the case of AAS with electrothermal atomization, 1.0 mL of a 1 per cent w/v solution of magnesium nitrate and 1.0 mL of a 10 per cent w/v solution of ammonium dihydrogen phosphate) and stabilizing agents may be used, if necessary.
BLANK SOLUTION Mix 4 mL of heavy metal-free hydrochloric acid and 6 mL of heavy metal-free nitric acid in a digestion flask. Carry out the digestion in the same manner as for the test solution.
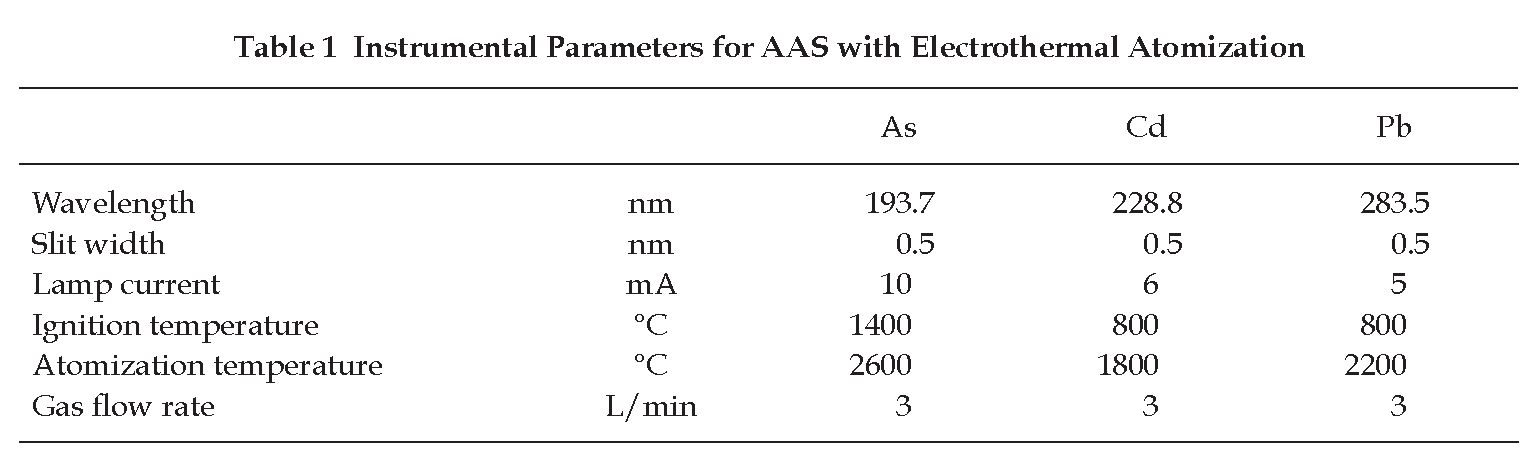
Determination of Arsenic, Cadmium and Lead Using AAS with Electrothermal Atomization
Carry out the test as described by the “Atomic Spectrometry: Emission and Absorption” (Appendix 2.3) and measure the content of arsenic, cadmium and lead by the “Method of Direct Calibration” (Method I, Appendix 2.3) or by the “Method of Standard Additions” (Method II, Appendix 2.3), using standard solutions of each heavy metal and the instrumental parameters recommended in Table 1. The absorbance value of the blank solution is subtracted from the value obtained with the test solution.
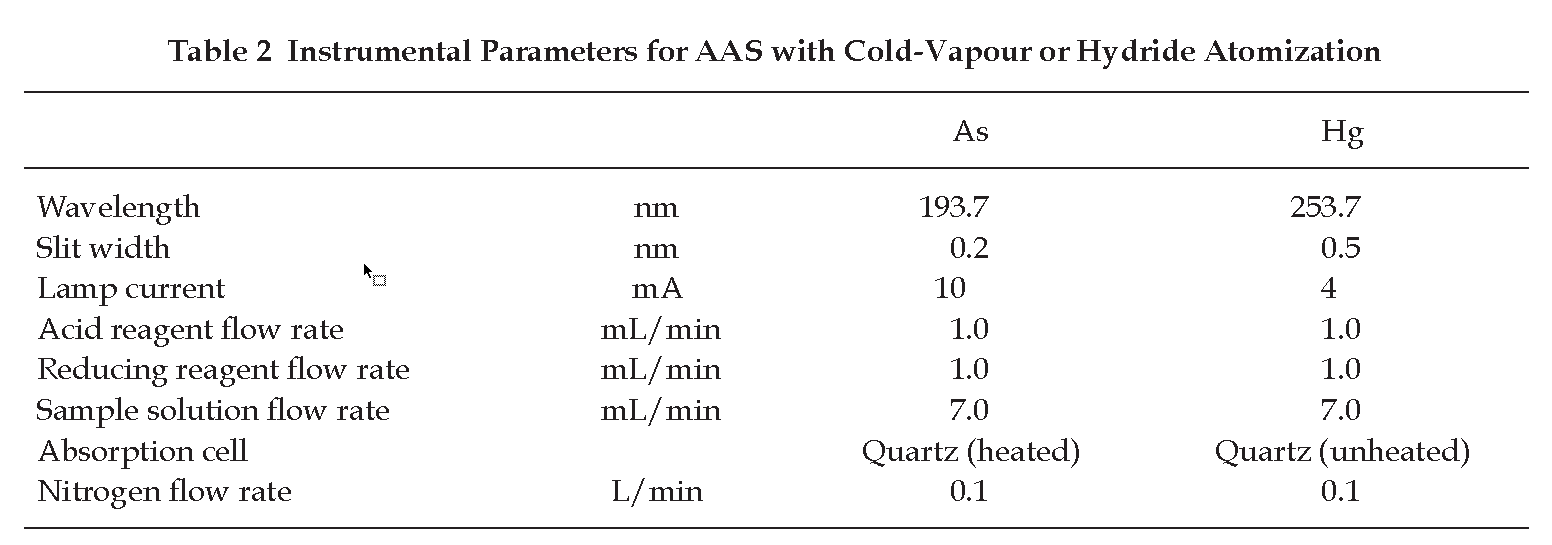
Determination of Arsenic, Cadmium and Lead Using AAS with Cold-Vapour or Hydride Atomization
Carry out the test as described by the “Atomic Spectrometry: Emission and Absorption” (Appendix 2.3) and measure the content of arsenic and mercury by the “Method of Direct Calibration” (Method I, Appendix 2.3) or by the “Method of Standard Additions” (Method II, Appendix 2.3), using standard solutions of arsenic or mercury and an automated continuous-flow hydride vapour generation system. The absorbance value of the blank solution is subtracted from the value obtained with the test solution.
ARSENIC
Sample solution To 19.0 mL of the test solution or of the blank solution as prescribed above, add 1 mL of a 20 per cent w/v solution of potassium iodide. Allow the test solution to stand at room temperature for about 50 minutes or at 70° for about 4 minutes.
Acid reagent Use Heavy metal-free hydrochloric acid.
Reducing reagent Prepare a 0.6 per cent w/v solution of sodium tetrahydroborate in a 0.5 per cent w/v solution of sodium hydroxide. The recommended instrumental parameters in Table 2 may be used.
MERCURY
Sample solution Use Test solution or Blank solution, as prescribed above.
Acid reagent Prepare a 51.5 per cent w/v solution of heavy metal-free hydrochloric acid.
Reducing reagent Prepare a 1.0 per cent w/v solution of stannous chloride in heavy metal-free dilute hydrochloric acid. The recommended instrumental parameters in Table 2 may be used.
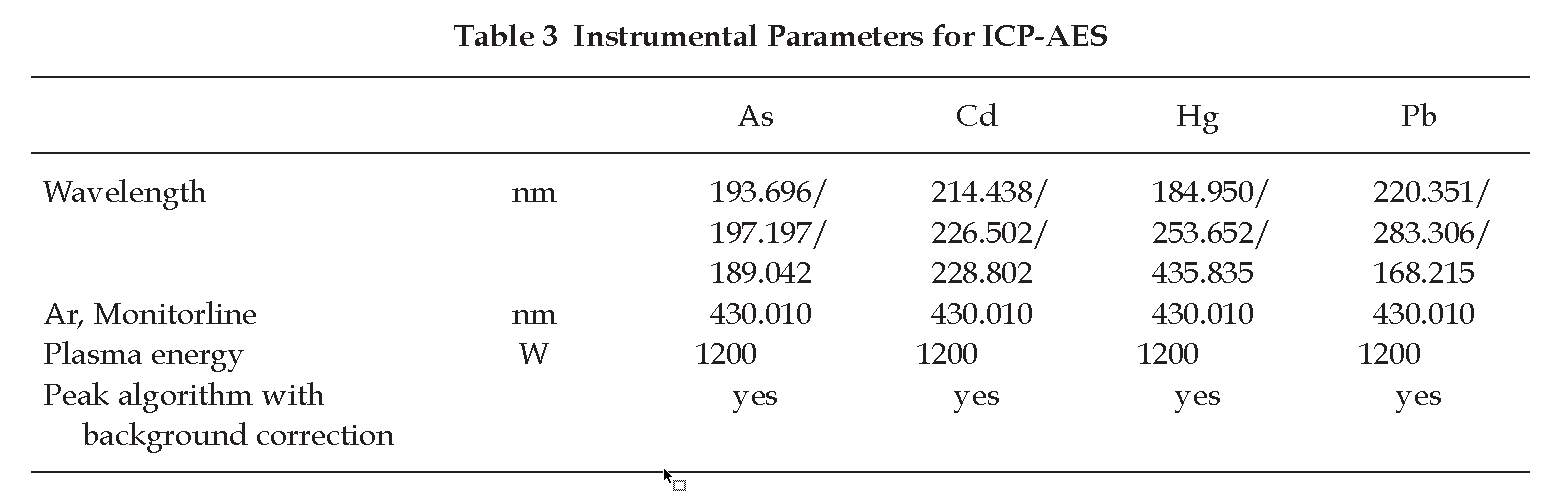
Determination of Arsenic, Cadmium, Mercury, and Lead Using ICP-AES
Carry out the test as described by the “ICP-AES”(Appendix 2.10) and measure the content of arsenic, cadmium, mercury, and lead by the “Method of Direct Calibration” (Method I, Appendix 2.3), using standard solutions of each heavy metal or a mixture of all measured metals, and the instrumental parameters recommended in Table 3.
The emission value of the blank solution is subtracted from the value obtained with the test solution.
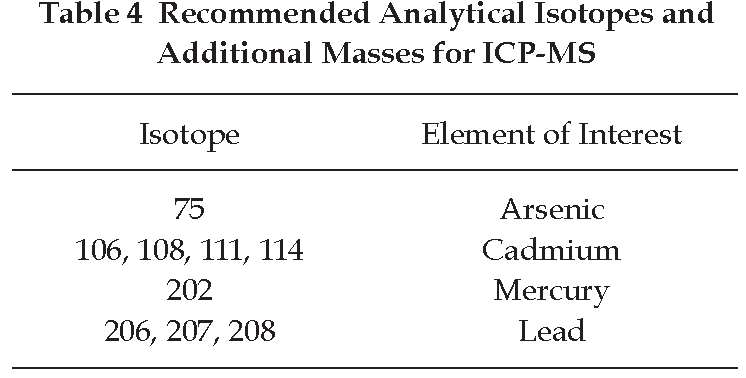
Determination of Arsenic, Cadmium, Mercury, and Lead Using ICP-MS
Carry out the test as described by the “ICP-MS” (Appendix 2.10) and measure the content of arsenic, cadmium, mercury, and lead by the “Method of Direct Calibration” (Method I, Appendix 2.3) using standard solutions of each heavy metal and the analytical isotopes and additional masses recommended in Table 4.
The signal intensity of the blank solution is subtracted from the value obtained with the test solution.
System Suitability
A system suitability test must be carried out on the day of the analysis to ensure that the sample preparation and measurement system are appropriate.
Acceptance criterion for preparation of sample solution A clear solution is obtained.
Acceptance criterion for measurement system The measured concentration of a standard solution of the metal at a concentration within the range of the used calibration curve does not differ from the actual concentration by more than 20 per cent.
Validation Requirements
The analytical procedures used must be validated in accordance with the relevant general methods as described in the “Atomic Spectrometry: Emission and Absorption” (Appendix 2.3), “ICP-AES”(Appendix 2.10) and “ICP-MS” (Appendix 2.10).
Additionally, the following criteria must be fulfilled.
Specificity
Specificity is the ability to ensure that the analytical procedures for sample preparation and measurement allow a reliable determination of the metal(s) in the presence of components (e.g., carrier gas, impurities, matrix) that may be expected to be
present.
Acceptance criteria The procedure must be able to assess unequivocally each heavy metal to be determined with this procedure in the presence of components that may be expected to be present, including other heavy metals, matrix components
and other sources of interference; specificity is demonstrated by complying with the accuracy requirement for the metal(s) to be determined.
Range
The calibration range of each metal is within the linear range of the method; test solutions containing residues at a level outside the calibration range may be diluted to concentrations within the calibration range.
Acceptance criterion Range is demonstrated by complying with the recovery requirement.
Accuracy
Verify the accuracy using a certified reference material or by performing a test for recovery.
RECOVERY Recovery may be determined on a sample of the substance to be examined, spiked with a known quantity of a reference standard of the metal (three concentration levels in the range of 50 to 150 per cent of the intended specification limit, even if the original concentration of the reference standard is at the specified value), in triplicate.
Acceptance criterion Spike recovery is within 70 per cent and 150 per cent for the mean of three replicates at each concentration.
Repeatability
TEST SAMPLES Either six independent samples of the substance to be examined spiked with a suitable reference standard at the specified concentration level, or three concentration levels prepared in triplicate.
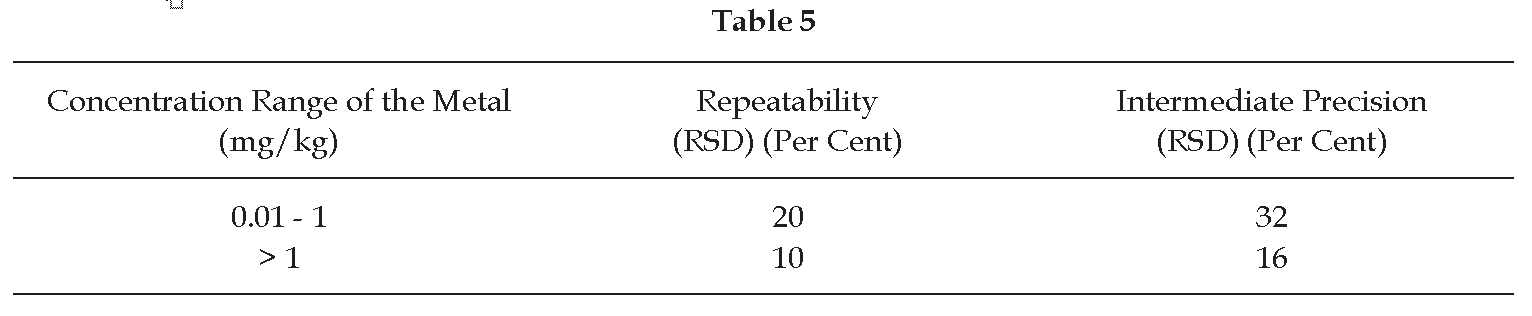
Acceptance criterion The relative standard deviation is in both cases not greater than the value indicated in Table 5.
Intermediate Precision
The effect of random events (intra-laboratory variations) on the analytical precision of the method must be established. Acceptable experiments for establishing intermediate precision include performing the repeatability analysis on different days, or with different instrumentation, or with different analysts. Only one of the three experiments is required to demonstrate intermediate precision.
Acceptance criterion The relative standard deviation is not greater than the value indicated in Table 5.
Limit of Quantification
Determine the lowest concentration meeting the acceptance criterion. Use the results from the accuracy study.
Acceptance criterion The limit of quantification is below the specification limit.
Limit of Detection (only Applicable to Limit Tests)
Determine the lowest concentration giving a signal clearly distinct from that obtained with a blank solution.
Acceptance criterion The limit of detection is not more than 0.1 times the concentration of the specification limit.